
In the dynamic and highly regulated world of clinical research, precision and efficiency are critical. From managing patient data to ensuring the right medication reaches the right participant, the complexities involved in modern trials demand innovative digital tools. Among these tools, RTSM software (Randomization and Trial Supply Management) has emerged as a vital component for managing trial logistics and data with accuracy and real-time control.
As pharmaceutical and biotech companies continue to design increasingly complex protocols, the ability to automate and streamline processes becomes not only helpful but essential. Randomization and drug supply logistics, once handled manually or through disconnected systems, can now be integrated into a single digital solution. This advancement enables clinical operations teams to reduce errors, respond faster to real-time developments, and maintain regulatory compliance more easily.

Understanding the Role of RTSM in Clinical Trials
Clinical trials involve numerous moving parts—patient enrollment, investigational product (IP) assignment, shipment tracking, and adherence to randomization protocols. RTSM software helps manage these aspects through a centralized, cloud-based platform. Its role extends to:
Automating patient randomization, ensuring unbiased treatment assignment.
Managing inventory levels across multiple trial sites.
Tracking and resupplying study drugs with real-time updates.
Offering secure access to trial data for authorized stakeholders.
Enhancing traceability and minimizing manual errors.
This system reduces the need for paper-based or spreadsheet-driven trial management methods. As a result, sponsors, CROs (Contract Research Organizations), and trial sites benefit from streamlined operations and faster decision-making.
Why Digital Integration Matters
Digital integration is more than a convenience—it's a necessity in today’s data-driven environment. Clinical studies produce vast volumes of data that must be accurately tracked, recorded, and analyzed. When the randomization and supply processes are managed through a disconnected or semi-automated method, risks increase—such as delays, data discrepancies, or protocol deviations.
With a well-designed RTSM system in place, trial sponsors can ensure seamless coordination between inventory logistics and patient dosing schedules. This ensures medications are delivered just-in-time and aligned with the study’s timeline, preventing potential interruptions or shortages.
Moreover, because clinical trials are conducted under strict regulatory scrutiny, RTSM platforms also offer audit trails, reporting features, and built-in compliance with international standards such as 21 CFR Part 11 and GDPR. This enables researchers and regulators alike to have full confidence in the data integrity.
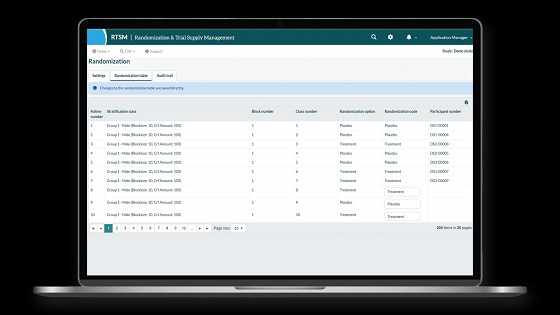
Key Benefits of Implementing RTSM Technology
Let’s explore the core benefits of using RTSM solutions in clinical trials:
1. Improved Randomization Accuracy
Traditional randomization methods can be prone to human error. RTSM tools use pre-configured algorithms, often designed in collaboration with trial statisticians, to ensure treatment assignments are randomized according to the protocol. This preserves the scientific integrity of the trial while minimizing bias.
2. Real-Time Drug Inventory Management
RTSM systems track drug supply at each site and enable automatic reorders or resupply alerts based on actual consumption rates. This predictive supply approach helps prevent stockouts and avoids drug wastage—common concerns in global studies.
3. Better Participant Experience
When medications arrive on time and clinical staff have access to real-time participant data, patient experiences improve. This, in turn, can enhance trial retention and data quality.
4. Faster Study Start-Up and Adaptability
RTSM solutions are typically built with configurable modules. This means that as trial parameters change—whether due to adaptive trial designs or regulatory amendments—the software can be quickly updated, minimizing downtime.
5. Enhanced Visibility and Control
Sponsors and CROs benefit from centralized dashboards that provide full visibility across sites. From monitoring enrollment progress to overseeing drug allocations and shipments, decision-makers have the data they need to act swiftly.
Challenges to Consider
While the advantages are clear, implementing RTSM solutions is not without its challenges. It requires upfront planning, thorough protocol understanding, and close coordination between technology providers and clinical teams. If misaligned, the software may not fully reflect the study’s randomization needs or supply logistics.
Furthermore, training for site personnel is crucial. Even the best system can fail if users are not adequately equipped to navigate it. Therefore, RTSM implementation should always include comprehensive onboarding, technical support, and feedback loops.
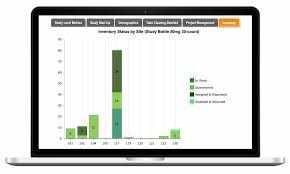
Trends in RTSM and the Future of Trial Management
As digital health continues to evolve, so does the functionality of RTSM tools. Here are a few emerging trends reshaping how randomization and supply management are handled:
Integration with EDC and eCOA: RTSM platforms are increasingly being connected with Electronic Data Capture (EDC) and electronic Clinical Outcome Assessment (eCOA) systems to offer a unified experience. This ensures consistent data flow and reduces duplicate entry.
AI-Driven Forecasting: Some advanced RTSM systems incorporate AI or machine learning to analyze usage patterns and improve drug supply predictions.
Mobile Accessibility: Investigators and monitors can access RTSM platforms via secure mobile apps or cloud portals, enabling on-the-go management and oversight.
Patient-Centric Designs: With the rise of decentralized clinical trials (DCTs), RTSM tools are adapting to include home delivery tracking, direct-to-patient shipping modules, and virtual visit management.
These trends point to a future where trial operations become more agile, efficient, and responsive to real-time needs, helping bring new treatments to market faster.
Making the Right Choice
When selecting an RTSM solution, clinical sponsors should focus on:
Flexibility and scalability
Regulatory compliance features
Integration capabilities
Real-time reporting
Quality of customer support
Security standards
Conducting a thorough vendor evaluation and involving end-users early in the selection process helps ensure the system is fit-for-purpose. Customizability is particularly important for adaptive or multi-arm trials where conditions may shift mid-study.
In a global clinical landscape marked by complexity, variability, and high costs, digital solutions like RTSM software are reshaping how clinical trials are executed. From reducing logistical bottlenecks to improving data accuracy, the impact of these systems is significant. Sponsors and CROs that invest in robust RTSM technology stand to benefit from smoother operations, faster timelines, and higher confidence in trial results. As the industry continues to evolve, RTSM platforms will play an even greater role in enabling smarter, more patient-centric research.






Write a comment ...